Understand which enzymes are responsible for the metabolism of your compound using recombinant enzymes.
The reaction phenotyping assay is one of our portfolio of in vitro ADME screening services. Cyprotex delivers consistent, high quality data with cost-efficiency that comes from a highly automated approach.
Introduction
Identification of enzymes (e.g. CYP450 and UGT) responsible for metabolism using recombinant enzymes:
- Cyprotex's Reaction Phenotyping assay uses expressed enzymes to identify which drug metabolizing isoforms are responsible for the metabolism of a test compound.
- Certain enzymes (e.g. CYPs) can be induced or exhibit polymorphisms which can greatly affect plasma levels in vivo therefore it is important to identify if this is expected to be a problem at an early stage.
- By identifying the enzyme responsible for the metabolism, it provides direction in identifying potential drug-drug interactions.
Protocol
Q & A
Please provide an overview of Cyprotex's reaction phenotyping assay
Test compound is incubated with expressed individual cytochrome P450 enzymes or uridine diphosphate glucuronosyl transferase enzymes at 37°C. For the cytochrome P450 reactions, the co-factor, NADPH is used to initiate the reaction. The reaction is terminated by solvent. Following centrifugation, the supernatant is analyzed by LC-MS/MS. The disappearance of test compound is monitored over a 45 minute time period. The test compound is also incubated in the presence of cDNA expressed control preparation (where no enzyme is present). The profile for the compound disappearance in the presence of the expressed enzyme is corrected for any disappearance observed in the presence of the control preparation. This ensures that only isoform-specific metabolism is determined. An example of a typical profile is detailed in Figure 1.
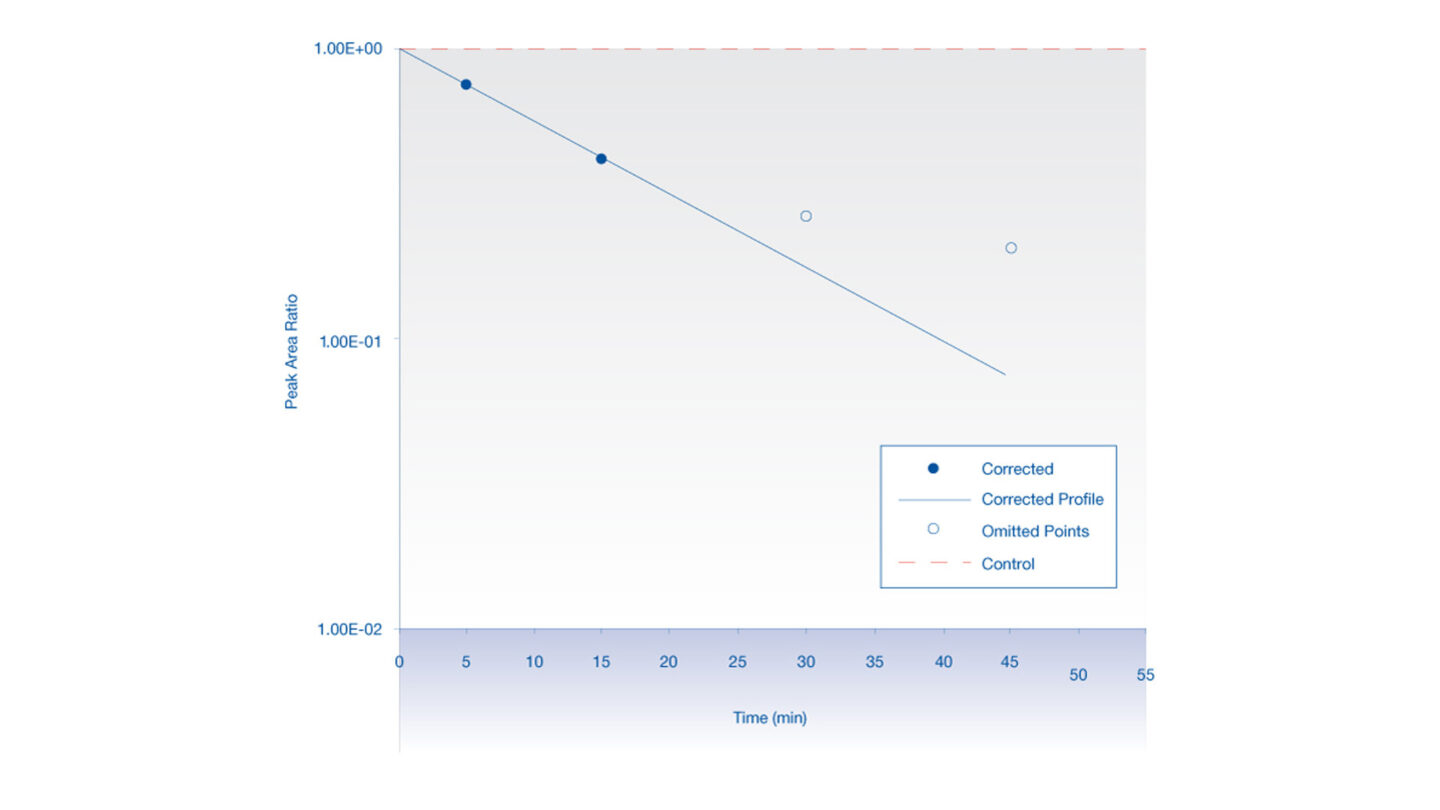
Figure 1
Parent compound disappearance with time in the presence of expressed enzyme (corrected for disappearance in the presence of cDNA expressed control preparation).
The peak area ratio (compound peak area/internal standard peak area) is plotted against time and the gradient of the line is determined.
The percent test compound remaining at each time point (after
correction for the disappearance in the presence of the cDNA expressed
control preparation) is also calculated and reported.
Why is it important to know which enzymes are involved in the metabolism of a drug?
There are several reasons why this screen is important. Firstly, the information may be used to predict potential drug-drug interactions with likely co-administered compounds. Secondly, certain cytochrome P450 enzymes are polymorphic (e.g., CYP2D6) and metabolism can vary considerably between individuals. If a compound is metabolized by an isoform which exhibits these characteristics, it may be detrimental, as plasma concentrations can vary considerably between subjects and determining an effective therapeutic range may be difficult.
The FDA guidance for drug interactions1 recommends using in vitro studies to determine if cytochrome P450 or uridine diphosphate glucuronosyl transferase enzymes are involved in the metabolism of a new investigational drug and its contribution to overall systemic clearance.
Cyprotex also offers a battery of tests to assess non CYP mediated metabolism or the effect of polymorphic metabolism.
How is the reaction phenotyping assay data used in drug discovery?
The data are interpreted in several ways. Firstly, clients may investigate the effect of structural changes on the extent of metabolism of the compound by a particular isoform. Secondly, if larger numbers of compounds are being screened, models can be built in order to predict whether a compound is likely to be metabolized by a particular isoform. Thirdly, it is important to evaluate the extent of contribution of individual CYP or UGT enzymes to meet current regulatory guidance.
Drug metabolizing systems which use cDNA expressed enzymes are artificial because the enzyme is not present in its native environment and is often over-expressed. With these systems, there is an absence of competing enzymes and reactions. However, a number of scaling approaches now exist, for example, the RAF (relative activity factor) method2 or more recently the ISEF (intersystem extrapolation factor) method3 which help to understand relative contribution of the individual enzymes.
Typically, the reaction phenotyping assay will be performed for compounds which show medium or high clearance in the microsomal or hepatocyte stability assays in order to understand the mechanism of metabolism, or if metabolism by a particular isoform has been considered to be a problem in the past.
References
1) FDA Guidance for Industry – In Vitro Drug Interaction Studies - Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions (January 2020)
2) Crespi CL. (1995) Advances in Drug Research 26; 179-235
3) Chen et al., (2011) Drug Metab Dispos 39(3); 373-382

