Introduction
- A highly purified population of cardiomyocytes, differentiated from human induced pluripotent stem (iPS) cells (Cellular Dynamics iCell® Cardiomyocytes), are used.
- The cells are a mixture of spontaneously electrically-active atrial, nodal, and ventricular-like myocytes. They possess typical human heart cell characteristics forming electrically connected syncytial layers that beat in synchrony, and exhibit expected electrophysiological and biochemical responses upon reference drug exposure.
- Viability is maintained for an extended culture periods (up to 2 weeks) allowing for acute and chronic studies.
- Microelectrode array (MEA) is one of the most sophisticated and efficacious technologies for measuring changes in spontaneously-active cells, such as cardiomyocytes and neurons.
- Cyprotex’s eCiphr®Cardio is a cell-based assay which uses MEA recording to monitor electrophysiological activity by measuring beat rate, field potential duration, amplitude and conduction velocity.
- Unlike the patch-clamp hERG assay, eCiphr®Cardio assesses changes in all major ion channels implicated in an action potential.
- This cardiac assay provides a unique in vitro system for preclinical drug discovery, cardiotoxicity assessment, disease modelling and high throughput phenotypic screening of drug candidates.
Protocol
eCiphr®Cardio Cell Based Assay Protocol
Data
Data from Cyprotex's eCiphr®Cardio Assay
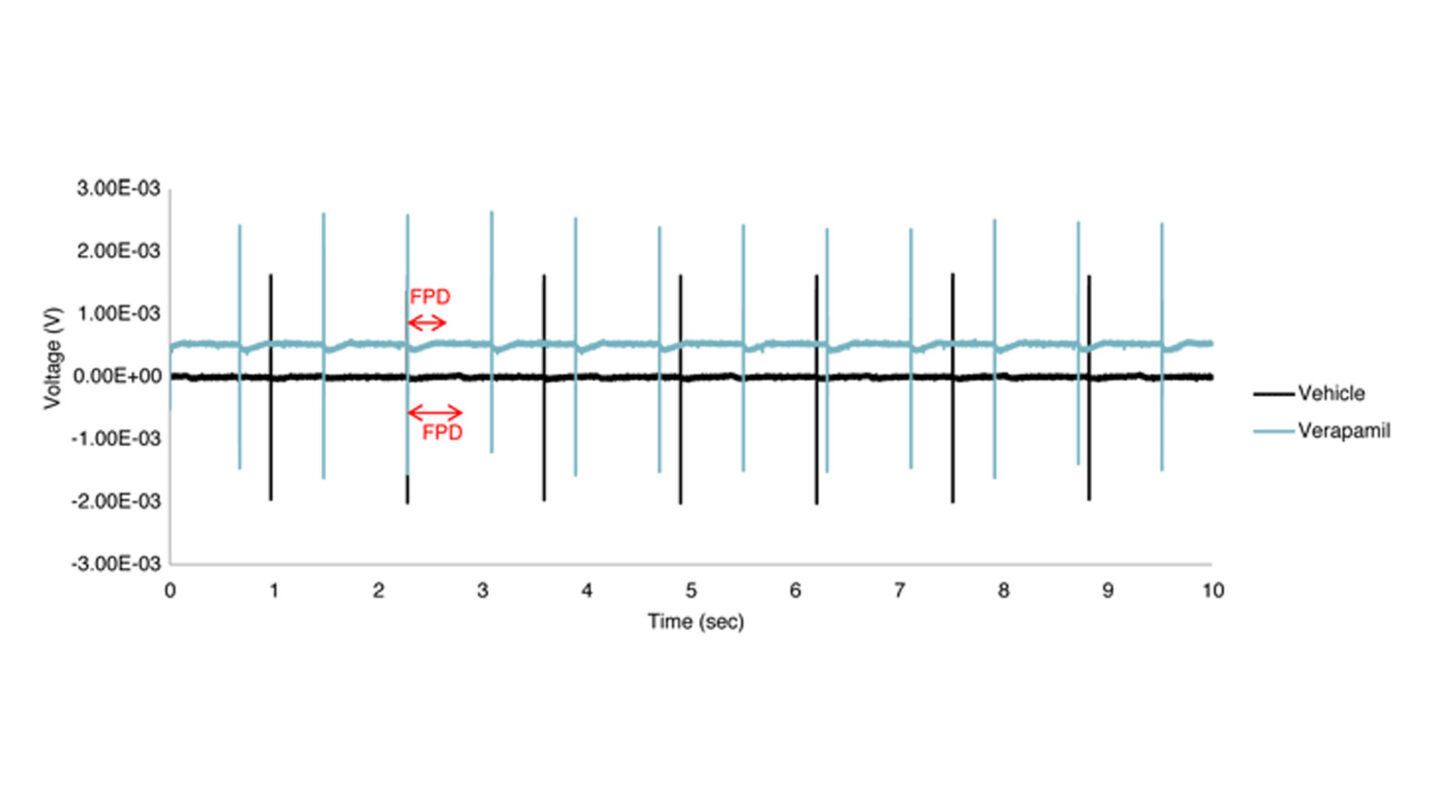
Figure 1
Raw traces for vehicle control (0.1% DMSO) and test compound (verapamil).
Red arrows point to the field potential duration (FPD, indicative of the QT interval duration). Verapamil clearly shortens FPD as compared to 0.1% DMSO (vehicle control). Note: In order to distinguish between the two traces, the voltage for verapamil is purposely shifted upward.
References
1) Wobus AM and Löser P. (2011) Arch Toxicol 85(2); 79-117
2) Peng S et al., (2010) J Pharmacol Toxicol Methods 61(3); 277-286
3) Finlayson K et al., (2004) Eur J Pharmacol 500(1-3); 129-142
4) Nattel S and Quantz MA, (1988) Cardiovasc Res 22(11); 808-817
5) Walker BD et al., (1999) Br J Pharmacol 128(2); 444-450
6) Zhang S et al., (1999) Cir Res 84; 989-998
7) Cheng HC and Incardona J, (2009) J Pharmacol Toxicol Methods 60(2); 174-184
8) Couderc JP et al., (2008) J Electrocardiol 41(6); 595-602
9) Rona G (1985) J Mol Cell Cardiol 17(4); 291-306
10) Paakkari I (2002) Toxicol Lett 127; 279-284

