Introduction
Drug-induced hemolysis is a relatively rare but serious toxicity liability. It occurs by two mechanisms1:
- Toxic hemolysis − direct toxicity of the drug, its metabolite, or an excipient in the formulation.
- Allergic hemolysis − toxicity caused by an immunological reaction in patients previously sensitized to a drug.
Cyprotex’s in vitro hemolysis testing is designed to screen for toxic hemolysis, as recommended by the US FDA.
Although the majority of normal individuals may suffer toxic hemolysis at sufficiently high concentrations of hemolytic drugs, for most drugs toxic hemolysis involves lower doses given to individuals who are genetically predisposed to hemolysis1.
The US FDA recommends that for excipients intended for injectable use, an in vitro hemolysis study should be performed at the intended concentration for IV administration to test for hemolytic potential2.
The in vitro hemolysis assay evaluates hemoglobin release in the plasma (as an indicator of red blood cell lysis) following test agent exposure.
Protocol
In Vitro Hemolysis Assay Protocol
Data
Data from Cyprotex's In Vitro Hemolysis Assay
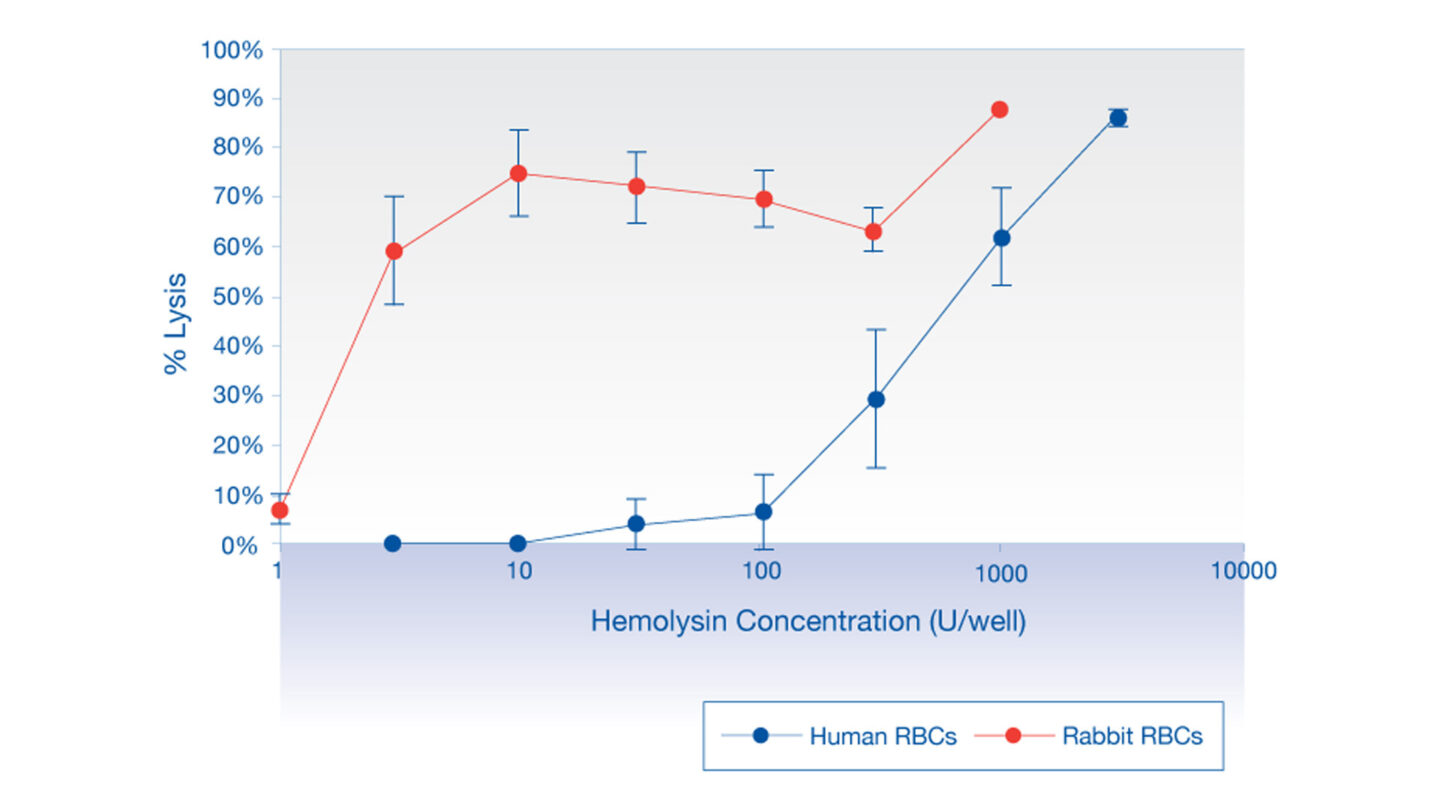
Figure 1
Hemolysis by hemolysin in human and rabbit erythrocytes.
Rabbit erythrocytes are much more susceptible to hemolysis by the bacterial exotoxin, hemolysin, than human erythrocytes, demonstrating the need to use human erythrocytes in hemolysis experiments.
Q&A
Please provide an overview of the in vitro hemolysis assay.
Serial dilutions of the compound are mixed with human blood and incubated for 45 minutes at 37°C. The cells are centrifuged and the absorbance of the supernatant, which includes plasma and lysed erythrocytes, is measured. Percent lysis is calculated from a standard curve of lysed erythrocytes.
Why is it important to investigate toxic hemolysis by drugs?
Exposure to high concentrations of drugs can cause hemolysis even in normal adults, but in individuals who are genetically predisposed to hemolysis, this can occur at lower concentrations. Neutropenia, thrombocytopenia, hemolytic anemia, aplastic anemia, and macrocytic anemia are known to occur in some patients treated with drugs3. Drugs, their metabolites, or excipients used in formulation can cause toxic hemolysis; therefore the FDA recommends testing for hemolytic potential2.
What controls are included in the in vitro hemolysis assay?
Positive control is Triton X-100, a known hemolytic agent4, 1% v/v and 0.1% v/v. Negative control is the vehicle (typically 0.5% DMSO).
How does in vitro hemolysis relate to in vivo drug-induced toxic hemolysis?
Cyprotex’s in vitro toxic hemolysis assay is a sensitive and accurate method for predicting toxic hemolysis of a drug. However, it should be noted that hemolysis sometimes occurs when blood is drawn. This should be taken into account when performing the assay. Also, coloured drugs that absorb light at the same wavelength at which the assay is measured (540 nm) can lead to false positives

